Chemistry, 05.05.2020 00:29 stefaniethibodeaux
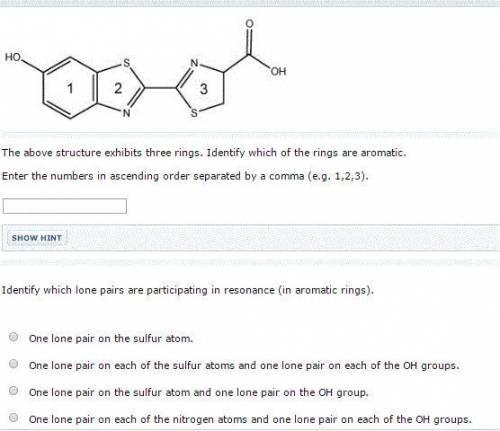
The above structure exhibits three rings. Identify which of the rings are aromatic. Enter the numbers in ascending order separated by a comma (e. g. 1,2,3). Identify which lone pairs are participating in resonance (in aromatic rings). One lone pair on each of the nitrogen atoms and one lone pair on each of the OH groups. One lone pair on the sulfur atom. One lone pair on the sulfur atom and one lone pair on the OH group. One lone pair on each of the sulfur atoms and one lone pair on each of the OH groups

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1
You know the right answer?
The above structure exhibits three rings. Identify which of the rings are aromatic. Enter the number...
Questions in other subjects:

Mathematics, 01.02.2021 17:40




Geography, 01.02.2021 17:40

Mathematics, 01.02.2021 17:40




Health, 01.02.2021 17:40