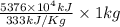On a hot summer day you and some friends decide you want to cool down your pool. Determine the mass of ice you would need to add to bring the equilibrium temperature of the system to 300K. The pool contains 400,000 L (at a density of 1 kg/L) of water initially at 305K. Assume the ice is at 0°C (273K), the heat capacity of water is 4.2 J/(g*K), and the enthalpy of melting ice is 333 kJ/kg.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 08:20, pilarmonsivais
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 18:00, faithabossard
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
On a hot summer day you and some friends decide you want to cool down your pool. Determine the mass...
Questions in other subjects:



Mathematics, 25.09.2021 05:50

Mathematics, 25.09.2021 05:50


Mathematics, 25.09.2021 05:50

Mathematics, 25.09.2021 05:50


English, 25.09.2021 05:50

Advanced Placement (AP), 25.09.2021 05:50

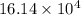 kg.
kg.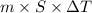
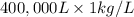
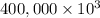 g (as 1 kg = 1000 g)
g (as 1 kg = 1000 g) = 305 K - 273 K
= 305 K - 273 K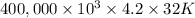
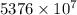 J
J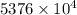 kJ
kJ