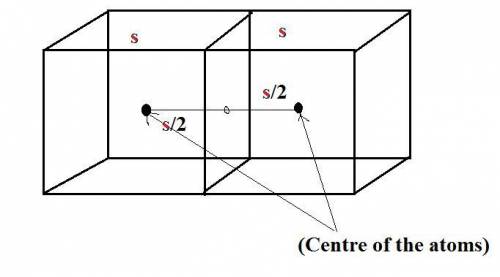
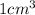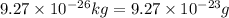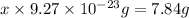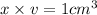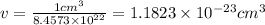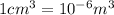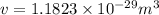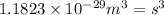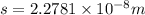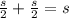Iron has a mass of 7.87 g per cubic centimeter of volume, and the mass of an iron atom is 9.27 × 10-26 kg. if you simplify and treat each atom as a cube, (a) what is the average volume (in cubic meters) required for each iron atom and (b) what is the distance (in meters) between the centers of adjacent atoms?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 21:30, steven0448
An atomic nucleus is composed ofa)protons. b)protons and neutrons. c)protons and electrons. d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 22.06.2019 22:30, COOLIOMARIS
What three things does a balanced equation show you?
Answers: 1
You know the right answer?
Iron has a mass of 7.87 g per cubic centimeter of volume, and the mass of an iron atom is 9.27 × 10-...
Questions in other subjects:





Mathematics, 01.12.2020 17:00




English, 01.12.2020 17:00

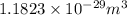 is the average volume required for each iron atom.
is the average volume required for each iron atom. .
.