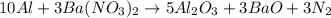Chemistry, 03.02.2020 14:00 trying2passs
In some fireworks there is a reaction between powdered aluminium and powdered barium nitrate in which heat is evolved and an unreactive gas is produced.
what is the equation for this reaction?
a. 2al + ba(no3)2 -> al2o3 + bao + 2no
b. 4al + 4ba(no3)2 -> 2al2o3 + 4 ba(no2)2 + o2
c. 10al + 3 ba(no3)2 -> 5al2o3 + 3bao + 3n2
d. 10al + 18ba(no3)2 -> 10al(no3)3 +18bao +3n2

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:30, ruleolivas
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 08:30, lpssprinklezlps
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3
You know the right answer?
In some fireworks there is a reaction between powdered aluminium and powdered barium nitrate in which...
Questions in other subjects:

Health, 23.10.2020 06:01

Mathematics, 23.10.2020 06:01






Social Studies, 23.10.2020 06:01