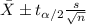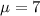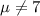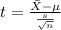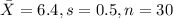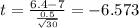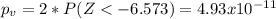Chemistry, 04.05.2020 22:37 lydia1melton
The Environmental Protection Agency has determined that safe drinking
water should have an average pH of 7 moles per liter. You are testing water from a new source, and take 30 vials of water. Water is unsafe if it deviates too far from 7 moles per liter in either direction. The mean pH level in your sample is 6.4 moles per liter, which is slightly acidic. The standard deviation of the sample is 0.5 moles per liter.
b) A 95% confidence interval for the true mean pH level of the water is (6.21, 6.59). Interpret this interval.
c) Explain why the interval in part (b) is consistent with the result of the test in part (a).

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, penelopymorales24
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1

Chemistry, 22.06.2019 08:40, alexisbcatlett14
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 16:40, westball101
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3
You know the right answer?
The Environmental Protection Agency has determined that safe drinking
water should have an ave...
water should have an ave...
Questions in other subjects:




Mathematics, 13.04.2021 03:00




World Languages, 13.04.2021 03:00