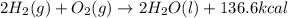Review energy changes by identifying the correct statements about each reaction.
2H2(g) +...

Chemistry, 03.05.2020 13:37 rahmao2773
Review energy changes by identifying the correct statements about each reaction.
2H2(g) + O2(g) ---> 2H2O(l) + 136.6 kcal
ANSWERS:
The reaction is: exothermic
The delta H value is: negative
Heat is a: product

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, tiniecisneros28
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1


Chemistry, 22.06.2019 05:40, yah2muchh
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 13:30, xojade
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 17.09.2020 07:01

Mathematics, 17.09.2020 07:01

Social Studies, 17.09.2020 07:01

Mathematics, 17.09.2020 07:01

Mathematics, 17.09.2020 07:01

Mathematics, 17.09.2020 07:01

Mathematics, 17.09.2020 07:01

Mathematics, 17.09.2020 07:01

Mathematics, 17.09.2020 07:01

Mathematics, 17.09.2020 07:01

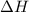 value is negative.
value is negative.