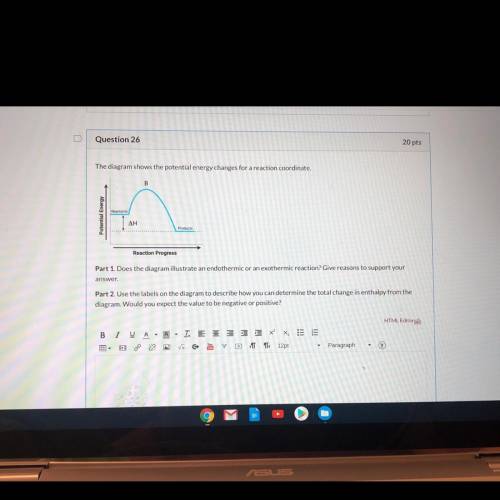
Part 1: Does the diagram illustrate an endothermic or an exothermic reaction?
Part 2: Use the...

Chemistry, 03.05.2020 13:07 locomexicano03
Part 1: Does the diagram illustrate an endothermic or an exothermic reaction?
Part 2: Use the labels on the diagram to describe how you can determine the total change in enthalpy from the diagram. Would you expect the value to be negative or positive?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 23:00, emilyphillips1681
If two identical atoms are bonded, what kind of molecule is formed
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 04.12.2021 20:40

Mathematics, 04.12.2021 20:40

Mathematics, 04.12.2021 20:40



Mathematics, 04.12.2021 20:40

Mathematics, 04.12.2021 20:40

French, 04.12.2021 20:40

Mathematics, 04.12.2021 20:40

Mathematics, 04.12.2021 20:40



