
Chemistry, 03.05.2020 13:04 Kaziyah461
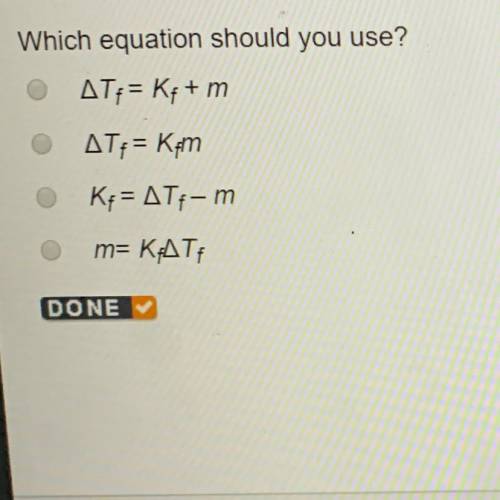
0.80 mol MgBr2 is added to 1.00 kg water. Determine the freezing point of the solution. Water has a
freezing point depression constant of 1.86°C. kg/mol.
Which equation should you use?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, nekathadon
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 14:30, Cartucho1978
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 20:50, iluminatioffial9699
One nanometer is equal to how many meters?
Answers: 2
You know the right answer?
0.80 mol MgBr2 is added to 1.00 kg water. Determine the freezing point of the solution. Water has a<...
Questions in other subjects:

Mathematics, 03.04.2020 06:24

Mathematics, 03.04.2020 06:24






Chemistry, 03.04.2020 06:24


Mathematics, 03.04.2020 06:24



