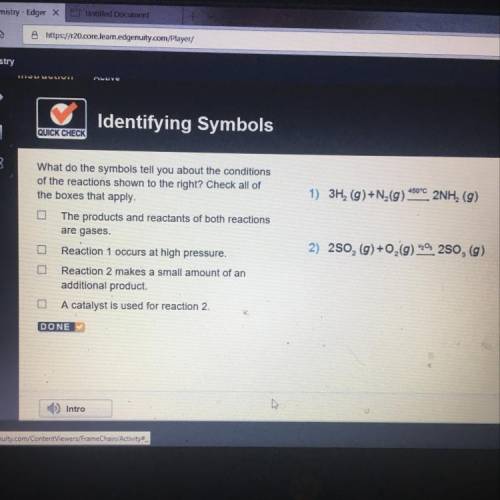
What do the symbols tell you about the conditions
of the reactions shown to the right? Check a...

Chemistry, 04.05.2020 23:25 christpress0
What do the symbols tell you about the conditions
of the reactions shown to the right? Check all of
the boxes that apply.
3H, (g) +N (9) 450°C 2NH, (g)
(2) 250, (g) + O2(g) Os 250, (g)
The products and reactants of both reactions
are gases.
Reaction 1 occurs at high pressure.
Reaction 2 makes a small amount of an
additional product.
A catalyst is used for reaction 2.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2

You know the right answer?
Questions in other subjects:

Biology, 26.06.2019 23:40





Geography, 26.06.2019 23:40


Mathematics, 26.06.2019 23:40

Mathematics, 26.06.2019 23:40




