
Chemistry, 05.05.2020 01:18 victoriavacodos
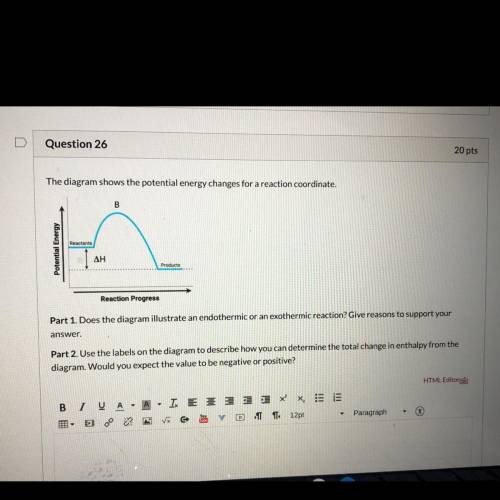
Part 1 : does the diagram illustrate an endothermic or exothermic reaction? Give reasons to support your answer.
Part 2: Use the labels on the diagram to describe how you can determine he total change in enthalpy from the diagram. Would you expect the value to be negative or positive?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, allyyzz
Astudent is given a sample of a blue copper sulfate hydrate. he weighs the sample in a dry covered porcelain crucible and got a mass of 23.875 g for the crucible, lid, and sample. the mass of the empty crucible and lid was found earlier to be 22.652 g. he then heats the crucible to expel the water of hydration, keeping the crucible at red heat for 10 minutes with the lid slightly ajar. on colling, he finds the mass of crucible, lid, and contents to be 23.403 g. the sample was changed in the process to very light clue anhydrous cuso4. if there are again 100.0 g of hydrate, how many grams of cuso4 are in it? how many moles of cuso4? (hint: molar mass of cuso4 = 159.6 g / mole. what per cent of the hydrate is cuso4? you may convert the mass of cuso4 to moles.)
Answers: 3

Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 09:00, oliviacolaizzi
Ineed to find the answer of this question because i dont understand it
Answers: 1
You know the right answer?
Part 1 : does the diagram illustrate an endothermic or exothermic reaction? Give reasons to support...
Questions in other subjects:

Chemistry, 23.03.2021 07:50




Mathematics, 23.03.2021 07:50

Arts, 23.03.2021 07:50

History, 23.03.2021 07:50

History, 23.03.2021 07:50

Biology, 23.03.2021 07:50




