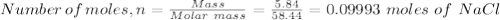A student has a mixture of salt (NaCl) and sugar (C12H22O11). To determine the percentage, the student measures out 5.84 grams of the mixture and dissolves this in 100.0 mL of water. The student knows that aqueous salt with aqueous silver nitrate (AgNO3) to form solid silver chloride (AgCl). The student determines that sugar will not react with silver nitrate. How many mL of 1.0 M AgNO3 would be required to precipitate 5.84 grams of NaCl?

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

You know the right answer?
A student has a mixture of salt (NaCl) and sugar (C12H22O11). To determine the percentage, the stude...
Questions in other subjects:










Mathematics, 16.04.2021 23:40