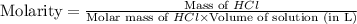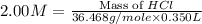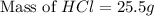Chemistry, 05.05.2020 03:17 aomoloju4202
An aqueous 2.00 M hydrochloric acid solution is prepared with a total volume of 0.350 L. The molecular
weight of hydrochloric acid is 36.468
mol
What mass of hydrochloric acid (in grams) is needed for the solution?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:50, toniawu18
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2

Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 23.06.2019 03:00, EllaLovesAnime
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1
You know the right answer?
An aqueous 2.00 M hydrochloric acid solution is prepared with a total volume of 0.350 L. The molecul...
Questions in other subjects:









English, 11.11.2020 17:50

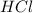 = 2.00 M
= 2.00 M