Chemistry, 05.05.2020 04:10 carterkanye468
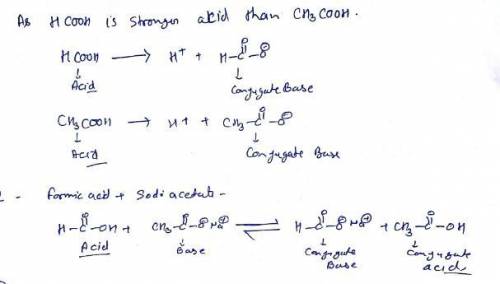
Given the following information: formic acid = HCOOH acetic acid = CH3COOH HCOOH is a stronger acid than CH3COOH (1) Write the net ionic equation for the reaction that occurs when equal volumes of 0.060 M aqueous formic acid and sodium acetate are mixed. It is not necessary to include states such as (aq) or (s). Use HCOO- as the formula for the formate ion.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:30, mathwiznot45
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1


Chemistry, 23.06.2019 09:00, hunterwilliams375
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
Answers: 2
You know the right answer?
Given the following information: formic acid = HCOOH acetic acid = CH3COOH HCOOH is a stronger acid...
Questions in other subjects:

Mathematics, 16.04.2021 05:30

History, 16.04.2021 05:30

Geography, 16.04.2021 05:30


Chemistry, 16.04.2021 05:30

Biology, 16.04.2021 05:30


Mathematics, 16.04.2021 05:30


Mathematics, 16.04.2021 05:30