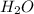Chemistry, 05.05.2020 04:06 asuhdude57
A website promoting the use of alternative energy vehicles and hybrid technologies claims that "A typical automobile in the USA uses about 40 gal of gasoline every month, producing about 750 lb of carbon dioxide." To determine the truth of this statement, calculate how many pounds of carbon dioxide are produced when 40.00 gal of gasoline are combusted. Assume that the primary ingredient in gasoline is octane, C8H18(l), which has a density of 0.703 g·mL−1.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, kevinh2683
Apump contains 0.5 l of air at 203 kpa. you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
A website promoting the use of alternative energy vehicles and hybrid technologies claims that "A ty...
Questions in other subjects:

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Chemistry, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

Mathematics, 16.09.2020 18:01

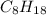 + 25
+ 25 ------------> 16
------------> 16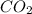 + 18
+ 18