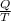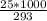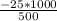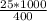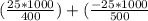Chemistry, 05.05.2020 05:40 olejlund8073
An object at 20∘C absorbs 25.0 J of heat. What is the change in entropy ΔS of the object? Express your answer numerically in joules per kelvin. ΔS = nothing J/K Request Answer Part E An object at 500 K dissipates 25.0 kJ of heat into the surroundings. What is the change in entropy ΔS of the object? Assume that the temperature of the object does not change appreciably in the process. Express your answer numerically in joules per kelvin. ΔS = nothing J/K Request Answer Part F An object at 400 K absorbs 25.0 kJ of heat from the surroundings. What is the change in entropy ΔS of the object? Assume that the temperature of the object does not change appreciably in the process. Express your answer numerically in joules per kelvin. ΔS = nothing J/K Request Answer Part G Two objects form a closed system. One object, which is at 400 K, absorbs 25.0 kJ of heat from the other object, which is at 500 K. What is the net change in entropy ΔSsys of the system? Assume that the temperatures of the objects do not change appreciably in the process. Express your answer numerically in joules per kelvin.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, thebrain1345
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 22.06.2019 20:30, sydneip6174
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 22.06.2019 23:10, carmenguabaoql9kv
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium. b)heavier than helium. c)the same weight as helium. d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
An object at 20∘C absorbs 25.0 J of heat. What is the change in entropy ΔS of the object? Express yo...
Questions in other subjects:


Business, 24.03.2021 17:40




Mathematics, 24.03.2021 17:40

Biology, 24.03.2021 17:40

English, 24.03.2021 17:40

Mathematics, 24.03.2021 17:40

History, 24.03.2021 17:40