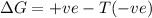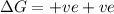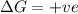Chemistry, 05.05.2020 06:34 Ryleetarver
Use the reaction data in the table below to select the answer choice that best describes this reaction.
Reaction Enthalpy Change
345.7 kJ/mol
Reaction Entropy Change
-25. 3 J/molK
This reaction is never spontaneous.
This reaction is spontaneous at all temperatures.
This reaction is spontaneous at low temperatures.
This reaction is spontaneous at high temperatures

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, jasminortega2002
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 00:30, rscott2649
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 10:10, jojomgarcia01
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
Use the reaction data in the table below to select the answer choice that best describes this reacti...
Questions in other subjects:


Mathematics, 20.11.2020 14:00


Mathematics, 20.11.2020 14:00


Physics, 20.11.2020 14:00


Mathematics, 20.11.2020 14:00

English, 20.11.2020 14:00

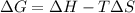
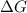 = Gibb's free energy change
= Gibb's free energy change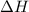 = enthalpy change
= enthalpy change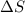 = entropy change
= entropy change