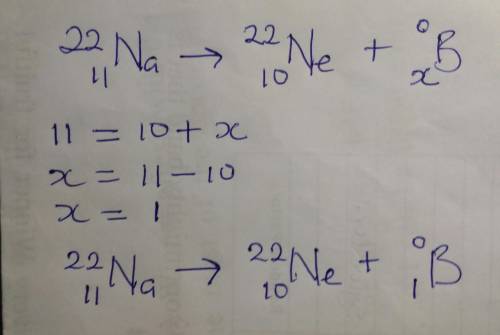Chemistry, 05.05.2020 06:03 kritalewis
Consider the nuclear equation below. Superscript 22 subscript 11 upper N a right arrow superscript 22 subscript 10 upper N e plus superscript 0 subscript question mark beta. Which is the missing value that will balance the equation?
-1
0
+1
+2

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, andersonemma2222
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


Chemistry, 22.06.2019 18:00, meowmeowcow
Find the mass, in grams, of 5.00*10^23 molecules of f2
Answers: 3
You know the right answer?
Consider the nuclear equation below. Superscript 22 subscript 11 upper N a right arrow superscript 2...
Questions in other subjects:


Mathematics, 08.12.2020 17:00

Mathematics, 08.12.2020 17:00






Mathematics, 08.12.2020 17:00