
Chemistry, 05.05.2020 05:58 CoolRahim9090
G Copper (II) Sulfate forms several hydrates with the general formula CuSO4 times xH2O, where x is an integer. If the hydrate is heated, the water can be drive off leaving pure CuSO4 behind. Suppose a sample of a certain hydrate is heated until all water is removed, and its found that the mass of the sample decreases by 31%. Which hydrate is it? THat is, WHAT IS X?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, deidaraXneji
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 21:00, taylorlanehart
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 22.06.2019 23:10, ArielA13
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3

Chemistry, 23.06.2019 05:30, victoria6929
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3
You know the right answer?
G Copper (II) Sulfate forms several hydrates with the general formula CuSO4 times xH2O, where x is a...
Questions in other subjects:


Mathematics, 23.06.2019 02:20

Mathematics, 23.06.2019 02:20


Chemistry, 23.06.2019 02:20



Mathematics, 23.06.2019 02:30

History, 23.06.2019 02:30

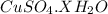
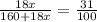
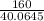 = 3.99 ≅ 4.
= 3.99 ≅ 4.


