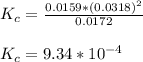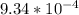Chemistry, 05.05.2020 07:20 stphdrn4347
Onsider the reversible dissolution of lead(II) chloride. P b C l 2 ( s ) − ⇀ ↽ − P b 2 + ( a q ) + 2 C l − ( a q ) PbClX2(s)↽−−⇀PbX2+(aq)+2ClX−(aq) Suppose you add 0.2393 g of P b C l 2 ( s ) PbClX2(s) to 50.0 mL of water. When the solution reaches equilibrium, you find that the concentration of P b 2 + ( a q ) PbX2+(aq) is 0.0159 M and the concentration of C l − ( a q ) ClX−(aq) is 0.0318 M. What is the value of the equilibrium constant, Kc, for the dissolution of P b C l 2 PbClX2?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, dustinquiz255
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3


Chemistry, 22.06.2019 07:30, isalih7256
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2
You know the right answer?
Onsider the reversible dissolution of lead(II) chloride. P b C l 2 ( s ) − ⇀ ↽ − P b 2 + ( a q ) + 2...
Questions in other subjects:



Advanced Placement (AP), 12.12.2020 17:00



English, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00


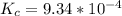
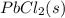 ⇌
⇌ 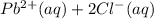
![[Pb^{2+}]](/tpl/images/0634/7278/0acfd.png) = 0.0159 M
= 0.0159 M
![[Cl^-]](/tpl/images/0634/7278/0726e.png) = 0.0318 M
= 0.0318 M
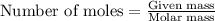
 Number of moles of PbCl₂
Number of moles of PbCl₂ 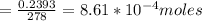
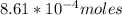
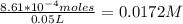
![K_c=\frac{[Pb^{2+}][Cl^-]}{[PbCl_2]} \\\\](/tpl/images/0634/7278/c4946.png)