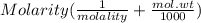An aqueous solution is 3.23M in tartaric acid (C4H06). The solution's density is 1.023 g/mL.
C...

Chemistry, 05.05.2020 09:09 biggiecheese93
An aqueous solution is 3.23M in tartaric acid (C4H06). The solution's density is 1.023 g/mL.
Calculate the solution's molality in tartaric acid.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, morrisjillian23
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 09:50, bridgetosanders
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 12:00, winterblanco
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 14:50, alexabbarker9781
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
You know the right answer?
Questions in other subjects:



Mathematics, 09.03.2021 19:00

Mathematics, 09.03.2021 19:00

Social Studies, 09.03.2021 19:00



Mathematics, 09.03.2021 19:00


Mathematics, 09.03.2021 19:00