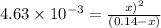The equilibrium constant for the reaction
COCl2 (g) CO (g) + Cl2 (g) is Kc = 4.63 ´ 10–3 at 52...

Chemistry, 05.05.2020 08:58 Kategaldamez3
The equilibrium constant for the reaction
COCl2 (g) CO (g) + Cl2 (g) is Kc = 4.63 ´ 10–3 at 527 °C
If 10 g of COCl2(g) is placed in a 1 L container, determine how much Cl2 is present at equilibrium.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, aidengalvin20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 04:50, shonnybenskin8
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 06:00, jwood287375
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3
You know the right answer?
Questions in other subjects:

English, 26.01.2020 05:31


English, 26.01.2020 05:31



Biology, 26.01.2020 05:31




Social Studies, 26.01.2020 05:31

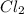 at equilibrium is 0.023 M
at equilibrium is 0.023 M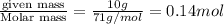
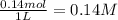
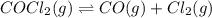
![K_c=\frac{[CO]\times [Cl_2]}{[COCl_2]}](/tpl/images/0635/7088/d6aec.png)