
Chemistry, 05.05.2020 11:05 mckinneypaige8243
A 14.3 g sample of HF is dissolved into 250 mL of solution. The concentration of the solution is *
A. 2.86 M
B. 0.14 M
C. 7.1 M
D. 3.6 M

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 09:40, kolibeilfuss
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 15:50, Edwardwall
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1
You know the right answer?
A 14.3 g sample of HF is dissolved into 250 mL of solution. The concentration of the solution is *
Questions in other subjects:


History, 08.01.2021 19:20




History, 08.01.2021 19:20

History, 08.01.2021 19:20

Mathematics, 08.01.2021 19:20

History, 08.01.2021 19:20

Mathematics, 08.01.2021 19:20


 ).
).

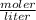 =2.86 M
=2.86 M

