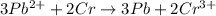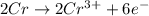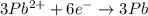Chemistry, 05.05.2020 13:14 madiiiiiii69
Given the balanced ionic equation:
3Pb2+(aq) + 2Cr(s) ---> 3Pb(s) + 2Cr3+(aq)
what is the number of moles of electrons gained by 3.0 moles of lead ions?
1. 5.0 mol
2. 2.0 mol
3. 3.0 mol
4. 6.0 mol

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 16:30, jrfranckowiak
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1


Chemistry, 22.06.2019 21:00, melissalopez12
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1
You know the right answer?
Given the balanced ionic equation:
3Pb2+(aq) + 2Cr(s) ---> 3Pb(s) + 2Cr3+(aq)
wh...
3Pb2+(aq) + 2Cr(s) ---> 3Pb(s) + 2Cr3+(aq)
wh...
Questions in other subjects:

Chemistry, 27.08.2020 20:01

English, 27.08.2020 20:01



English, 27.08.2020 20:01


Biology, 27.08.2020 20:01


Mathematics, 27.08.2020 20:01