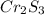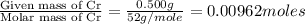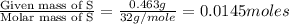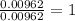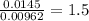Chemistry, 05.05.2020 15:08 teionamwhite2262
A 0.500-g sample of chromium metal reacted with sulfur powder to give 0.963 g of product. Calculate the empirical formula of the chromium sulfide.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, hannacarroll2539
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 22.06.2019 10:00, alexabdercmur
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 10:00, valdezlizbeth6652
Why is carbon ideal for making different compounds?
Answers: 2

Chemistry, 22.06.2019 13:00, cnfndbxbfbdb2031
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
A 0.500-g sample of chromium metal reacted with sulfur powder to give 0.963 g of product. Calculate...
Questions in other subjects:

Mathematics, 16.12.2020 18:50


Chemistry, 16.12.2020 18:50

Mathematics, 16.12.2020 18:50



Mathematics, 16.12.2020 18:50

Mathematics, 16.12.2020 18:50

Mathematics, 16.12.2020 18:50

Physics, 16.12.2020 18:50