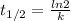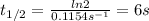Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, laurachealsy923
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 22.06.2019 03:40, jude3412
In an effort to address concerns about global warming, a power plant in portland, oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1


Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3
You know the right answer?
A → B is a first order reaction. Using the data below, what is the half-life in seconds? 0 sec, [A]...
Questions in other subjects:

Chemistry, 18.03.2021 05:20


Mathematics, 18.03.2021 05:20


Mathematics, 18.03.2021 05:20

Mathematics, 18.03.2021 05:20

Mathematics, 18.03.2021 05:20



Chemistry, 18.03.2021 05:20