
Chemistry, 05.05.2020 17:07 Natavia3402
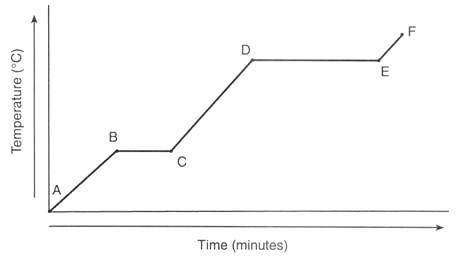
A 47.7 g chunck of ice at -58.0 °C is heated until it completely melts. Find the total amount of heat in joules for this process to occur. = 2.09J/g°C
= 2.03 J/g°C
= 4.184 J/g°C
∆ = 334 J/g
∆ = 2260 J/g
What is the total amount of energy needed to overall kilojoules (three significant figures)?
A. 21.7 kJ
B. 5.78 kJ
C. 15.9 kJ
D. 10.1

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, kkmonsterhigh18
The diagram below shows a cell placed in a solution. a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution. only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it. it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 09:00, wkalpakchi
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1


Chemistry, 22.06.2019 23:00, Mynameismath
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3
You know the right answer?
A 47.7 g chunck of ice at -58.0 °C is heated until it completely melts. Find the total amount of hea...
Questions in other subjects:


Biology, 20.01.2021 17:20

English, 20.01.2021 17:20

Physics, 20.01.2021 17:20

Mathematics, 20.01.2021 17:20


Biology, 20.01.2021 17:20

Business, 20.01.2021 17:30


Medicine, 20.01.2021 17:30



