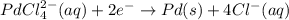Chemistry, 05.05.2020 18:31 cdradlet2001
A voltaic cell that uses the reaction PdCl42−(aq)+Cd(s) → Pd(s)+4Cl−(aq)+Cd2+(aq) has a measured standard cell potential of +1.03 V. You may want to reference (Pages 860 - 867) Section 20.4 while completing this problem. Part A Write the half-cell reaction at the cathode.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, kcarstensen59070
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 23:00, lufung8627
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 03:30, nikkio4
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus. b) the number of neutrons it contains in its nucleus. c) the number of protons it has in a cloud around the nucleus. d) the number of neutrons it has in a cloud around the nucleus. e) the number of electrons it exchanges with its neighbors.
Answers: 1

Chemistry, 23.06.2019 06:40, 1991987
1.) which of the following is a molecule but not a compound? a. he b. f2 c. h2o d. ch4 2.) what is a physical combination of substances? a. a compound b. a molecule c. a mixture d. an element 3.) what is a chemical combination of substances? a. a compound b. an atom c. a mixture d. an element 4.) what is the relationship between the solute and solvent in a solution? a. they form a compound b. they form a mixture c. they form molecules d. they form chemical bonds 5.) the gases in air dissolve in water. what would be one way to reduce the amount of a gas dissolved in water? a. add more water b. reduce the air pressure c. increase the air pressure d. stir the water 6.) how would you determine the solubility of a substance? a. find how well it dissolved various substances. b. find the mass and the volume of the substance. c. find the temperature at which the substance evaporated. d. find how much i was able to dissolve in a solute. 7.) the periodic table organizes all of the kinds of a. molecules. b. compounds. c. atoms. d. ions. 8.)what distinguishes two substances combined to become a compound vs. two substances combined to become a mixture? a. whether they can be easily separated b. whether they chemically bond together c. whether they both are visible d. whether they are heterogeneous 9.) the principle components of air are: n2 78% o2 21% ar 0.95% co2 0.038% this is a solution of a. molecules and atoms. b. molecules. c. compounds and molecules. d. atoms.
Answers: 1
You know the right answer?
A voltaic cell that uses the reaction PdCl42−(aq)+Cd(s) → Pd(s)+4Cl−(aq)+Cd2+(aq) has a measured sta...
Questions in other subjects:




Mathematics, 24.05.2020 21:58



Mathematics, 24.05.2020 21:58


History, 24.05.2020 21:58