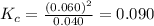Chemistry, 05.05.2020 18:04 AgentPangolin
At high temperatures, carbon reacts with O2 to produce CO as follows: C(s) O2(g) 2CO(g). When 0.350 mol of O2 and excess carbon were placed in a 5.00-L container and heated, the equilibrium concentration of CO was found to be 0.060 M. What is the equilibrium constant, Kc, for this reaction

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:30, thatonestudent2271
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1


Chemistry, 23.06.2019 03:00, kuehlthau03
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1
You know the right answer?
At high temperatures, carbon reacts with O2 to produce CO as follows: C(s) O2(g) 2CO(g). When 0.350...
Questions in other subjects:

Mathematics, 24.11.2019 10:31



Mathematics, 24.11.2019 10:31

Mathematics, 24.11.2019 10:31

Health, 24.11.2019 10:31

Mathematics, 24.11.2019 10:31


Mathematics, 24.11.2019 10:31

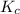 is 0.090.
is 0.090.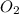 =
= 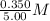 = 0.0700 M
= 0.0700 M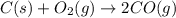
![K_{c}=\frac{[CO]^{2}}{[O_{2}]}](/tpl/images/0641/0273/4df57.png) , where [CO] and
, where [CO] and ![[O_{2}]](/tpl/images/0641/0273/9a638.png) represents equilibrium concentration of CO and
represents equilibrium concentration of CO and ![[CO]=2x=0.060](/tpl/images/0641/0273/522d6.png)