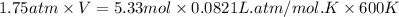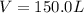Chemistry, 05.05.2020 21:22 estebencampos69
At 600 K and 1.75 atm, if 16.00 grams of H2 will reacts with excess N2 what volume in liters will be produced of NH3?
N2(g) + 3 H2(g) --> 2 NH3(g)
(R = 0.0821 L atm/mol K)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3


Chemistry, 22.06.2019 15:30, abdullaketbi71
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 23:00, NewKidnewlessons
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
You know the right answer?
At 600 K and 1.75 atm, if 16.00 grams of H2 will reacts with excess N2 what volume in liters will be...
Questions in other subjects:

Mathematics, 09.07.2019 15:00


History, 09.07.2019 15:00


History, 09.07.2019 15:00




Chemistry, 09.07.2019 15:00

Biology, 09.07.2019 15:00

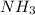 produced will be, 150.0 L
produced will be, 150.0 L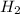
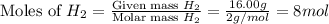
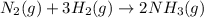
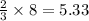 mole of
mole of