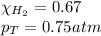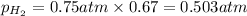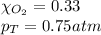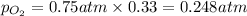Chemistry, 05.05.2020 22:21 ayoismeisjjjjuan
Solve the following, show all work and units for your calculation: Let's say we have a mixture of hydrogen gas
(H2), and oxygen gas (O2). The mixture contains 6.7 mol hydrogen gas and 3.3 mol oxygen gas. The mixture is
in a 300 L container at 273 K and the total pressure of the gas mixture is 0.75 atm. What is the partial
pressure for each gas?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, alexandroperez13
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3


Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3
You know the right answer?
Solve the following, show all work and units for your calculation: Let's say we have a mixture of hy...
Questions in other subjects:


Mathematics, 01.08.2019 05:30




Mathematics, 01.08.2019 05:30

Mathematics, 01.08.2019 05:40

History, 01.08.2019 05:40

Mathematics, 01.08.2019 05:40

History, 01.08.2019 05:40

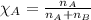
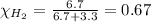
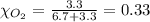
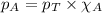
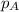 = partial pressure of substance
= partial pressure of substance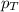 = total pressure
= total pressure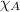 = mole fraction of substance
= mole fraction of substance