

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, stefaniethibodeaux
Which supports the idea that birds and butterflies both have wings but they do not have a common ancestor with wings? a. the wings are analogous structures that evolved differently and do not have a similar internal structure. b. the wings are homologous structures that evolved differently and do not have a similar internal structure. c. wings of birds are vestigial structures, but the wing structures of bats are not vestigial. d. wings of bats are vestigial structures, but the wing structures of birds are not vestigial
Answers: 1

Chemistry, 22.06.2019 09:40, kolibeilfuss
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

You know the right answer?
Compared to a 1.0-liter aqueous solution with a pH of 7.0, a 1.0-liter aqueous solution with a pH of...
Questions in other subjects:




History, 14.04.2020 07:25

Mathematics, 14.04.2020 07:25

Mathematics, 14.04.2020 07:25

Mathematics, 14.04.2020 07:25

Mathematics, 14.04.2020 07:25


Mathematics, 14.04.2020 07:26

![pH=-\log[H^+]](/tpl/images/0643/0989/cf945.png)
![pOH=-\log[OH^-]](/tpl/images/0643/0989/fe336.png)
![7=-\log[H^+]](/tpl/images/0643/0989/1fb8e.png)
![[H^+]=10^{-7}M](/tpl/images/0643/0989/0ed7f.png)
![5=-\log[H^+]](/tpl/images/0643/0989/54e2a.png)
![[H^+]=10^{-5}M](/tpl/images/0643/0989/34493.png)
![\frac{[H^+]_{pH=5}}{[H^+]_{pH=7}}=\frac{10^{-5}}{10^{-7}}\\\\\frac{[H^+]_{pH=5}}{[H^+]_{pH=7}}=10^2](/tpl/images/0643/0989/c09c8.png)
![[H^+]_{pH=5}=100\times [H^+]_{pH=7}](/tpl/images/0643/0989/ec551.png)
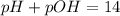
![7=-\log[OH^-]](/tpl/images/0643/0989/783d7.png)
![[OH^-]=10^{-7}M](/tpl/images/0643/0989/889ca.png)
![9=-\log[OH^-]](/tpl/images/0643/0989/7e7a8.png)
![[OH^-]=10^{-9}M](/tpl/images/0643/0989/f7c96.png)
![\frac{[OH^-]_{pH=7}}{[OH^-]_{pH=5}}=\frac{10^{-7}}{10^{-9}}\\\\\frac{[OH^-]_{pH=7}}{[OH^-]_{pH=5}}=10^2](/tpl/images/0643/0989/17ac9.png)
![[OH^-]_{pH=7}=100\times [OH^-]_{pH=5}](/tpl/images/0643/0989/65bd7.png)


