Given the general chemical equation 3A + 2B → C + 5D,
a. How many moles of D can be prod...


Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 23.06.2019 00:00, savyblue1724707
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3
You know the right answer?
Questions in other subjects:

Biology, 27.06.2020 01:01



Mathematics, 27.06.2020 01:01

Mathematics, 27.06.2020 01:01



English, 27.06.2020 01:01


Mathematics, 27.06.2020 01:01

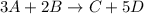
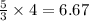 moles of D
moles of D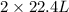 volume of B gives
volume of B gives 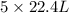 volume of D
volume of D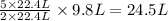 volume of D
volume of D

