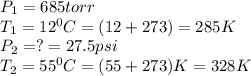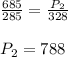A sample of ideal gas is in a sealed container. The pressure of the gas is 685 torr , and the temperature is 12 ∘C . If the temperature changes to 55 ∘C with no change in volume or amount of gas, what is the new pressure, P2, of the gas inside the container?Express your answer with the appropriate units.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, AleciaCassidy
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1


Chemistry, 22.06.2019 09:30, junkmailemail42
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1
You know the right answer?
A sample of ideal gas is in a sealed container. The pressure of the gas is 685 torr , and the temper...
Questions in other subjects:

Chemistry, 20.01.2021 09:10

Mathematics, 20.01.2021 09:10


Mathematics, 20.01.2021 09:10

English, 20.01.2021 09:10

Arts, 20.01.2021 09:10

Mathematics, 20.01.2021 09:10


Mathematics, 20.01.2021 09:10

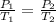
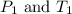 are the initial pressure and temperature of the gas.
are the initial pressure and temperature of the gas.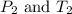 are the final pressure and temperature of the gas.
are the final pressure and temperature of the gas.