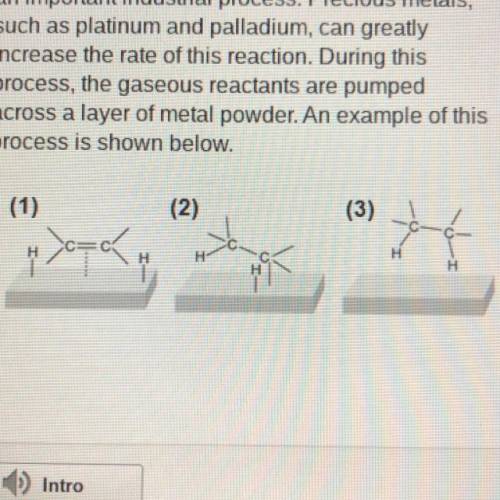
The hydrogenation of unsaturated hydrocarbons is
an important industrial process. Precious met...

Chemistry, 05.05.2020 22:59 linacelina6027
The hydrogenation of unsaturated hydrocarbons is
an important industrial process. Precious metals,
such as platinum and palladium, can greatly
increase the rate of this reaction. During this
process, the gaseous reactants are pumped
across a layer of metal powder. An example of this
process is shown below.
Describe how the metal probably increases the
reaction rate, identify whether this is an example of
homogeneous or heterogeneous catalysis, and
explain how you know.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 19:50, mikaylaaaaa
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 23.06.2019 00:10, graceception
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3

Chemistry, 23.06.2019 01:30, rubyr9975
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1
You know the right answer?
Questions in other subjects:



English, 11.02.2021 05:40

Mathematics, 11.02.2021 05:40

History, 11.02.2021 05:40


Mathematics, 11.02.2021 05:40


Mathematics, 11.02.2021 05:40



