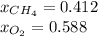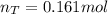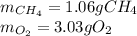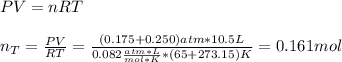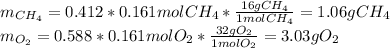Chemistry, 06.05.2020 00:28 andrwisawesome0
The partial pressure of CH4(g) is 0.175 atm, and the partial pressure of O2(g) is 0.250 atm in a mixture of the two gases. The mixture occupies a volume of 10.5 L at 65 oC. Solve all three parts of the question. 1) The mole fraction of CH4(g) is , and the mole fraction of O2(g) is . 2) The total number of moles of gas in the mixture is . 3) There are grams of CH4(g) and grams of O2(g) in the mixture.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, bbrogle5154
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
The partial pressure of CH4(g) is 0.175 atm, and the partial pressure of O2(g) is 0.250 atm in a mix...
Questions in other subjects:



Mathematics, 23.12.2020 18:40


Mathematics, 23.12.2020 18:40

Mathematics, 23.12.2020 18:40


Social Studies, 23.12.2020 18:40