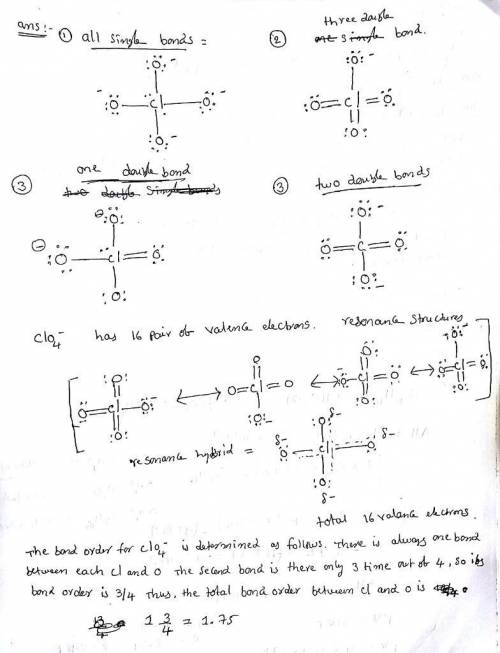
Erchlorates are powerful oxidizing agents used in fireworks, flares, and space shuttle booster rockets. Lewis structures for the perchlorate ion (ClO4−) can be drawn with all single bonds or with one, two, or three double bonds. Draw each of these possible resonance forms, including any nonbonding electrons. Include the values of any nonzero formal charges. Use formal charges to determine the most important resonance structure and calculate its average bond order.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:20, maevemboucher78
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 18:00, sandeebassett3
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
You know the right answer?
Erchlorates are powerful oxidizing agents used in fireworks, flares, and space shuttle booster rocke...
Questions in other subjects:

Mathematics, 10.02.2021 21:00


Mathematics, 10.02.2021 21:00


Mathematics, 10.02.2021 21:00



Geography, 10.02.2021 21:00

Mathematics, 10.02.2021 21:00