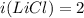Two aqueous solutions are prepared: 1.00 m Na2CO3 and 1.00 m LiCl. Which of the following statements is true?
A. The Na2CO3 solution has a higher osmotic pressure and higher vapor pressure than the LiCl solution.
B. The Na2CO3 solution has a higher osmotic pressure and higher boiling point than the LiCl solution.
C. The Na2CO3 solution has a lower osmotic pressure and lower vapor pressure than the LiCI solution.
D. The Na2CO3 solution has a lower osmotic pressure and higher boiling point than the LiCl solution.
E. None of these statements is true.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, penelopymorales24
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1

Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 23.06.2019 07:00, ultimatesaiyan
Introduction of drugs into the gastrointestinal tract is a form of administration. a. enteral b. topical c. parenteral d. inhalation
Answers: 1

Chemistry, 23.06.2019 08:30, lowkstahyna
What percentage of energy used in the u. s is produced from fossil fuels
Answers: 2
You know the right answer?
Two aqueous solutions are prepared: 1.00 m Na2CO3 and 1.00 m LiCl. Which of the following statements...
Questions in other subjects:

Physics, 10.07.2019 07:30

Mathematics, 10.07.2019 07:30



Social Studies, 10.07.2019 07:30





Geography, 10.07.2019 07:30

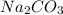 solution has a higher osmotic pressure and higher boiling point than LiCl solution.
solution has a higher osmotic pressure and higher boiling point than LiCl solution.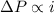 ,
, 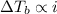 and
and 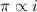
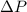 ,
, 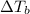 and
and 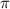 are lowering of vapor pressure, elevation in boiling point and osmotic pressure of solution respectively.
are lowering of vapor pressure, elevation in boiling point and osmotic pressure of solution respectively.  is van't hoff factor.
is van't hoff factor.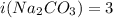 and
and