Chemistry, 06.05.2020 00:15 dpinzoner5952
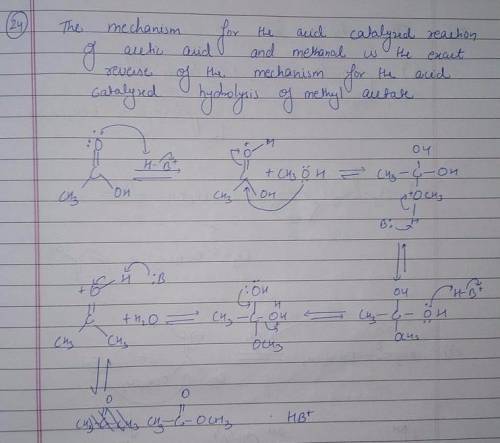
Using the mechanism for the acid-catalyzed hydrolysis of an ester as your guide, write the mechanism-showing all the curved arrows-for the acid-catalyzed reaction of acetic acid and methanol to form methyl acetate. All proton-donating and proton-removing species are given on canvas.
there are 7 boxesO
||
the first one has H3C-C-OH with OH+ --H
O
||
and the final product is H3C-C-O-CH3 with H3O+

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, kimberlyrios12p0ts98
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 09:30, janetexcoelho
What does the mass of 0.7891 mol of ferric oxide (fe2o3)
Answers: 1
You know the right answer?
Using the mechanism for the acid-catalyzed hydrolysis of an ester as your guide, write the mechanism...
Questions in other subjects:


Mathematics, 18.01.2020 18:31


Biology, 18.01.2020 18:31


Physics, 18.01.2020 18:31

Chemistry, 18.01.2020 18:31

English, 18.01.2020 18:31

Mathematics, 18.01.2020 18:31