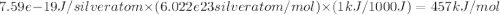The work function (ϕ) of a metal is the minimum energy needed to remove an electron from its surface. (a) Is it easier to remove an electron from a gaseous silver atom or from the surface of solid silver (ϕ = 7.59×10−19 J; IE = 731 kJ/mol)? (b) Explain the results in terms of the electron-sea model of metallic bonding.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:10, ChaseRussell24
Atank contains 240 liters of fluid in which 10 grams of salt is dissolved. brine containing 1 gram of salt per liter is then pumped into the tank at a rate of 6 l/min; the well-mixed solution is pumped out at the same rate. find the number a(t) of grams of salt in the tank at time t.
Answers: 3

Chemistry, 22.06.2019 09:00, boxergirl2062
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 21:30, starl0rd211
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 22.06.2019 23:00, lufung8627
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
The work function (ϕ) of a metal is the minimum energy needed to remove an electron from its surface...
Questions in other subjects:


Mathematics, 25.07.2020 04:01

Advanced Placement (AP), 25.07.2020 04:01



Mathematics, 25.07.2020 04:01


Physics, 25.07.2020 04:01