
Methanol, is made by using a catalyst to react carbon monoxide with hydrogen at high temperature and pressure. Assuming that 450.0 of CO and 825 of H2O are allowed to react, answer the following questions. (Hint: First write the balanced chemical equation for this reaction.)
Which reactant is in excess?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, poopybutt541
Avariable that is not being directly tested during an experiment should be
Answers: 1



Chemistry, 23.06.2019 00:30, hdhshshs741
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1
You know the right answer?
Methanol, is made by using a catalyst to react carbon monoxide with hydrogen at high temperature and...
Questions in other subjects:


English, 05.05.2020 05:21


Mathematics, 05.05.2020 05:21


Mathematics, 05.05.2020 05:21




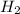 are allowed to react, answer the following questions. (Hint: First write the balanced chemical equation for this reaction.)
are allowed to react, answer the following questions. (Hint: First write the balanced chemical equation for this reaction.)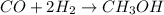
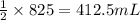 of carbon monoxide
of carbon monoxide


