
Chemistry, 06.05.2020 03:11 acavalieri72
Predict whether ΔS for each reaction would be greater than zero, less than zero, or too close to zero to decide.
ΔS > 0; ΔS < 0; too close to decide
I2(g) + Cl2(g) > 2ICl(g)
2NOBr(g) > 2NO(g) + Br2(g)
CO2(g) + H2(g) > CO(g) + H2O(g)
2H2O2(I) > 2H2O(I) + O2(g)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, luffybunny
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1


Chemistry, 22.06.2019 19:00, andrecoral105
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 23.06.2019 00:20, jessicamcummins
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
Predict whether ΔS for each reaction would be greater than zero, less than zero, or too close to zer...
Questions in other subjects:

Biology, 23.07.2019 01:50






English, 23.07.2019 01:50



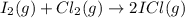 : too close to decide.
: too close to decide.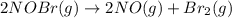 :
: 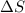 > 0.
> 0.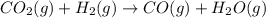 : too close to decide.
: too close to decide.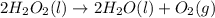 :
: 

