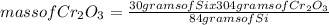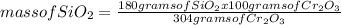Chemistry, 06.05.2020 04:26 desteyness8178
Chromium(III) oxide (Cr2O3) reacts with silicon (Si) as indicated by the balanced equation below:
2Cr2O3(s) + 3Si(s) — 4Cr(s) + 3SiO2(s)
Determine the mass of SiO2 (60.08 g mol-4) when 30.00 g of Si reacts with 100.0 g of Cr2O3 (152 0 g mol-1).

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 20:30, camerondillonn
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 23.06.2019 04:00, clickbaitdxl
What two categories of toxins were present in the air at dish, texas as a result of the gas pipelines that pass through the area
Answers: 1
You know the right answer?
Chromium(III) oxide (Cr2O3) reacts with silicon (Si) as indicated by the balanced equation below:
Questions in other subjects:



Chemistry, 12.03.2021 21:20

English, 12.03.2021 21:20


Mathematics, 12.03.2021 21:20

Chemistry, 12.03.2021 21:20

Mathematics, 12.03.2021 21:20