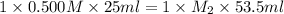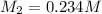Chemistry, 05.02.2020 08:02 sascsl2743
In a titration, 25.0 ml of 0.500 m khp is titrated to the equivalence point with naoh. the final solution volume is 53.5 ml. what is the value of m2 for the reaction?
0.439 m
0.500 m
0.234 m
1.07 m

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 20:30, ashley4329
Select all the correct answers. which compounds have the empirical formula ch20? (multiple answers)a. c2h4o2b. c3h603c. ch2o2d. c5h1005e. c6h1206
Answers: 2

Chemistry, 22.06.2019 22:30, xlebrny7831
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
In a titration, 25.0 ml of 0.500 m khp is titrated to the equivalence point with naoh. the final sol...
Questions in other subjects:





Mathematics, 15.01.2021 20:30





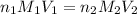
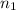 = basicity of an acid = 1
= basicity of an acid = 1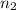 = acidity of a base = 1
= acidity of a base = 1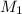 = concentration of KHP = 0.500 M
= concentration of KHP = 0.500 M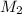 = concentration of NaOH = ?
= concentration of NaOH = ?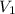 = volume of KHP = 25 ml
= volume of KHP = 25 ml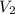 = volume of NaOH = 53.5 ml
= volume of NaOH = 53.5 ml