Chemistry, 06.05.2020 04:11 addisonrausch
One reaction involved in the conversion of iron ore to the metal is FeO(s) + CO(g) → Fe(s) + CO2(g) Use Hess’s Law to calculate ΔHrxn given the following. 3 Fe2O3(s) + CO(g) → 2 Fe3O4(s) + CO2(g) ΔH = −47.0 k Fe2O3(s) + 3 CO(g) → 2 Fe(s) + 3 CO2(g) ΔH = −25.0 k Fe3O4(s) + CO(g) → 3 FeO(s) + CO2(g) ΔH = 19.0 k

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 20:00, emilyswinge4421
Listenbase your answer to the question on the information below. nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body. cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment. which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2


You know the right answer?
One reaction involved in the conversion of iron ore to the metal is FeO(s) + CO(g) → Fe(s) + CO2(g)...
Questions in other subjects:


Geography, 10.10.2019 19:00

Biology, 10.10.2019 19:00

Mathematics, 10.10.2019 19:00


Mathematics, 10.10.2019 19:00


Spanish, 10.10.2019 19:00


Biology, 10.10.2019 19:00

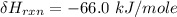
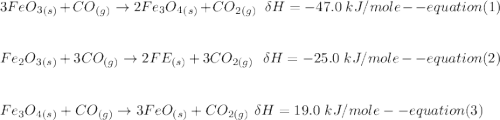
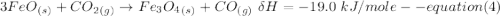
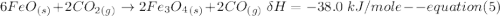
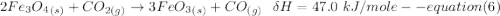


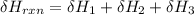 (According to Hess Law)
(According to Hess Law)