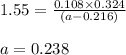Chemistry, 06.05.2020 04:03 Benjamincompton07
The equilibrium constant, Kc, for the following reaction is 1.55 at 667 K.
2NH3(g) <> N2(g) + 3H2(g)
When a sufficiently large sample of NH3(g) is introduced into an evacuated vessel at 667 K, the equilibrium concentration of H2(g) is found to be 0.324 M.
Calculate the concentration of NH3 in the equilibrium mixture.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1


Chemistry, 22.06.2019 10:30, esnyderquintero
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 13:30, xojade
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1
You know the right answer?
The equilibrium constant, Kc, for the following reaction is 1.55 at 667 K.
2NH3(g) <...
2NH3(g) <...
Questions in other subjects:

Biology, 13.07.2021 03:00

English, 13.07.2021 03:00


Mathematics, 13.07.2021 03:00

Mathematics, 13.07.2021 03:00


History, 13.07.2021 03:00

Mathematics, 13.07.2021 03:00

History, 13.07.2021 03:00

History, 13.07.2021 03:00

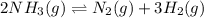
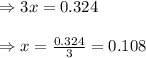
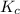 for above equation follows:
for above equation follows:![K_c=\frac{[N_2][H_2]^3}{[NH_3]^2}](/tpl/images/0646/0178/a64ad.png)