

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, fgcherubin
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 22:30, kristen17diaz
How many valence electrons are in atom of radon?
Answers: 1

Chemistry, 23.06.2019 14:20, shaylawaldo11
Neutral atoms of argon, atomic number 18, have the same number of electrons as each of the following items except: cl- s -2 k+ ca +2 ne
Answers: 2
You know the right answer?
Glycine has pKa values of 2.34 and 9.60. Therefore, glycine will exist predominately as a zwitterion...
Questions in other subjects:




Mathematics, 25.09.2021 17:20



Chemistry, 25.09.2021 17:20


Mathematics, 25.09.2021 17:20

Mathematics, 25.09.2021 17:20

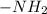 and -COOH should bear equal and opposite charges in it's zwitterionic structure.
and -COOH should bear equal and opposite charges in it's zwitterionic structure.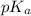 of -COOH (in glycine) = 2.34
of -COOH (in glycine) = 2.34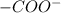 .
.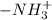 .
.

