
Standard solution of FeSCN2+FeSCN2+ is prepared by combining 9.09.0 mL of 0.200.20 M Fe(NO3)3Fe(NO3)3 with 1.01.0 mL of 0.00200.0020 M KSCN. KSCN. The equation for the reaction is as follows. Fe(NO3)3+KSCN↽−−⇀FeSCN2++KNO3+2NO−3 Fe(NO3)3+KSCN↽−−⇀FeSCN2++KNO3+2NO3− What allows us to assume that the reaction goes essentially to completion? The reaction quotient Q is greater than Kc. Kc. The concentration of Fe(NO3)3Fe(NO3)3 is much higher than the concentration of KSCN. KSCN. The excess Fe3+Fe3+ prevents the formation of the neutral Fe(SCN)3.Fe(SCN)3. The equlibrium reaction has a very high Kc. Kc. Under the conditions given, Le Châtelier's principle dictates that the reaction shifts to the left. Based on that assumption, what is the equilibrium concentration of FeSCN2+?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, fvmousdiana
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 22:30, safiyabrowne7594
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1

You know the right answer?
Standard solution of FeSCN2+FeSCN2+ is prepared by combining 9.09.0 mL of 0.200.20 M Fe(NO3)3Fe(NO3)...
Questions in other subjects:

History, 14.04.2020 23:25


History, 14.04.2020 23:25


Mathematics, 14.04.2020 23:25






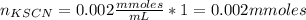
![[Fe(NO3)3]=\frac{1.8}{10} =0.18M](/tpl/images/0646/9896/2543f.png)
![[KSCN]=\frac{0.002}{10} =0.0002M](/tpl/images/0646/9896/5abd0.png)



