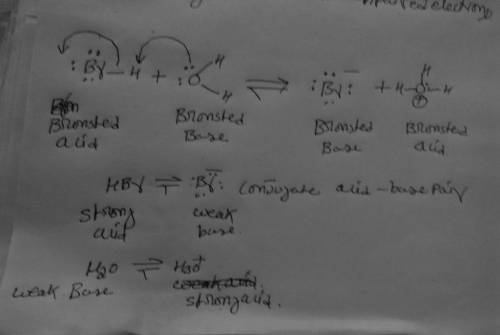
The wet cotton traps any HBr that escapes the reaction mixture. The trapping reaction is simply what happens when HBr dissolves in water, but it is still an acid/base reaction that is in principle reversible. Complete the acid/base equilibrium started for you below, draw the curved arrow pushing in BOTH directions and identify the stronger and weaker Bronsted acid and base on each side of the equilibrium.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, coryoddoc3685
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 10:10, dhailyortegacampa131
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 12:30, AnastasiaJauregui
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 16:30, Kathryn014
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
The wet cotton traps any HBr that escapes the reaction mixture. The trapping reaction is simply what...
Questions in other subjects: