
Chemistry, 06.05.2020 07:07 zitterkoph
Gaseous ammonia chemically reacts with oxygen o2 gas to produce nitrogen monoxide gas and water vapor. Calculate the moles of nitrogen monoxide produced by the reaction of 0.050mol of ammonia . Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, EinsteinBro
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 09:00, alydiale584
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 10:40, justicejesusfreak
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 23.06.2019 03:30, uniqueray33
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
You know the right answer?
Gaseous ammonia chemically reacts with oxygen o2 gas to produce nitrogen monoxide gas and water vapo...
Questions in other subjects:

English, 22.06.2019 18:30

English, 22.06.2019 18:30

History, 22.06.2019 18:30

Social Studies, 22.06.2019 18:30


English, 22.06.2019 18:30

Social Studies, 22.06.2019 18:30


History, 22.06.2019 18:30

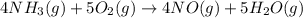
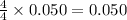 moles of nitrogen monoxide
moles of nitrogen monoxide

