
Suppose a 250.mL flask is filled with 0.70mol of H2 and 1.6mol of HCl. The following reaction becomes possible:
H2 (g) + Cl2 (g) <---> 2HCl (g)
The equilibrium constant K for this reaction is 0.262 at the temperature of the flask. Calculate the equilibrium molarity of HCl. Round your answer to two decimal places.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, noathequeen
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 23.06.2019 06:00, tytianadyson74
What volume of argon gas is equal to 1.60 grams of argon
Answers: 1


Chemistry, 23.06.2019 08:00, vetterk1400
Drag each pressure unit with the corresponding number to describe standard atmospheric pressure
Answers: 1
You know the right answer?
Suppose a 250.mL flask is filled with 0.70mol of H2 and 1.6mol of HCl. The following reaction become...
Questions in other subjects:



History, 26.07.2019 03:30

Biology, 26.07.2019 03:30


History, 26.07.2019 03:30


History, 26.07.2019 03:30

Social Studies, 26.07.2019 03:30

![\frac{[HCl]^2}{[H_{2}][Cl_{2}]}](/tpl/images/0648/3167/ae53b.png) = 0.262
= 0.262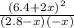 x = 0.285
x = 0.285 

